

Using the above calculator you could find that e.g. Or 1 mole of a substance will contain Avogadro's number of that substance. Be able to find the number of atoms or molecules in a given weight of a substance. Define molecular weight, formula weight, and molar mass explain how the latter differs from the first two. The term " mole" is defined in that one mole of a substance with a molecular (or atomic) mass of one (1), will have a mass of 1 gram. Be able to calculate the number of moles in a given mass of a substance, or the mass corresponding to a given number of moles. Magnesium Carbonate molecular weight Molar mass of MgCO3 84. It is defined to be 1/12 of the mass of one atom of carbon-12 and in older works is also abbreviated as "amu".Īlso, important in this field is Avogadro's number (N A) or Avogadro's constant (6.0221 x 10 23). In related terms, another unit of mass often used is Dalton (Da) or unified atomic mass unit (u) when describing atomic masses and molecular masses. Molecular mass or molar mass are used in stoichiometry calculations in chemistry. This Calculator has been tested on Internet Explorer version 6 only,įirefox might not show all fields correctly. For question or remarks please contact us. !!! Lenntech BV cannot be held responsible for errors in the calculation, Make sure you enter the molecule of crystallization at last (e.g. The calculator handles at most two different bracket levels. The molecular mass calculator will recognize the entered formula's, which are included in the list of organic compounds.
#MAGNESIUM MOLAR WEIGHT SERIES#
Or you can choose by one of the next two option-lists, which contains a series of common organic compounds (including their chemical formula) and all the elements. This online calculator you can use for computing the average molecular weight (MW) of molecules by entering the chemical formulas (for example C3H4OH(COOH)3 ). Molecular Weight Calculator Molecular Weight Calculator

Express each of the events A, B, C, and D as sets of ordered pairs that are subsets of S.Ĭ.
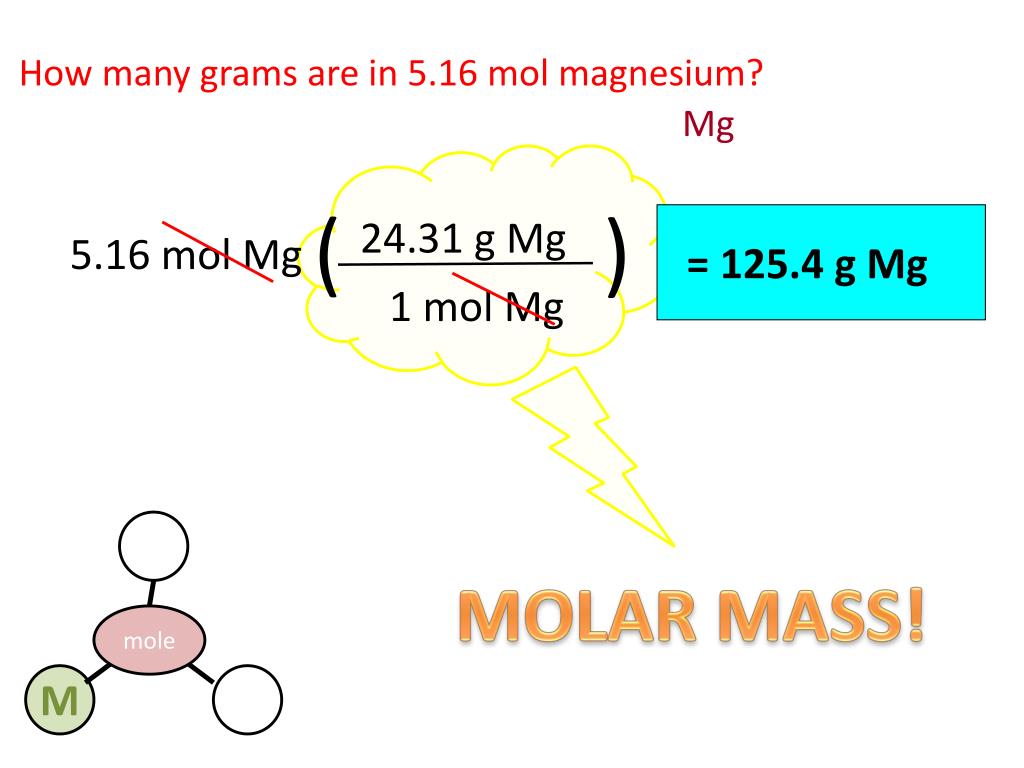
Define a sample space S for the experiment as a set of ordered pairs that makes it possible for you to express the four sets above as events.ī. Answer and Explanation: 1 Become a member to unlock this answer Create your account View this answer Given Data: Molar mass of magnesium is 24 g.
Let A be the event that the power cell is functional, let B be the event that two subcells have the same voltage, let C be the event that the first subcell has a strictly higher voltage than the second subcell, and let D be the event that the power cell is not functional but needs less than one additional volt to become functional.Ī. An experiment consists of measuring and recording the voltages of the two subcells. The power cell is functional if and only if the sum of the two voltages of the subcells is at least 6 volts. A power cell consists of two subcells, each of which can provide from 0 to 5 volts, regardless of what the other subcell provides.


 0 kommentar(er)
0 kommentar(er)
